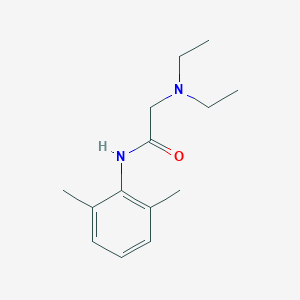
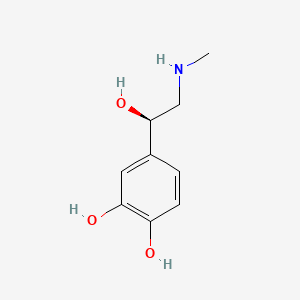
Caudal block, Lumbar epidural block
Adult: Available preparation for epidural test dose:
Lidocaine 1.5% (15 mg) and epinephrine 1:200,000 (5 mcg) per mL solution for inj
Administer test dose (2-3 mL) at least 5 minutes prior to injecting the total volume required for a lumbar or caudal block.
Available preparations for epidural anaesthesia:
Lidocaine 1% (10 mg) and epinephrine 1:200,000 (5 mcg) per mL solution for inj
Lidocaine 1.5% (15 mg) and epinephrine 1:200,000 (5 mcg) per mL solution for inj
Lidocaine 2% (20 mg) and epinephrine 1:200,000 (5 mcg) per mL solution for inj
Dosage varies with the number of dermatomes to be anaesthetised (generally 2-3 mL/dermatome). Max: Lidocaine 7 mg/kg (not to exceed 500 mg). For continuous epidural or caudal anaesthesia, Max dose should not be administered at intervals of <90 minutes. Dosage recommendations may vary among countries and individual products (refer to specific product guidelines).
Lidocaine 1.5% (15 mg) and epinephrine 1:200,000 (5 mcg) per mL solution for inj
Administer test dose (2-3 mL) at least 5 minutes prior to injecting the total volume required for a lumbar or caudal block.
Available preparations for epidural anaesthesia:
Lidocaine 1% (10 mg) and epinephrine 1:200,000 (5 mcg) per mL solution for inj
Lidocaine 1.5% (15 mg) and epinephrine 1:200,000 (5 mcg) per mL solution for inj
Lidocaine 2% (20 mg) and epinephrine 1:200,000 (5 mcg) per mL solution for inj
Dosage varies with the number of dermatomes to be anaesthetised (generally 2-3 mL/dermatome). Max: Lidocaine 7 mg/kg (not to exceed 500 mg). For continuous epidural or caudal anaesthesia, Max dose should not be administered at intervals of <90 minutes. Dosage recommendations may vary among countries and individual products (refer to specific product guidelines).
Parenteral
Dental infiltration and dental nerve block
Adult: Available preparations:
Lidocaine 2% (20 mg) and epinephrine 1:100,000 (10 mcg) per mL solution for inj
Lidocaine 2% (20 mg) and epinephrine 1:80,000 (22.7 mcg) per mL solution for inj
Dose is individualised based on the physical status of the patient, the vascularity of the oral tissues, the area of the oral cavity to be anaesthetised, and the technique of anaesthesia used. For oral infiltration or mandibular block: 1-5 mL. Max: Lidocaine 7 mg/kg (not to exceed 200-500 mg, depending on the procedure and product used). Dosage recommendations may vary among countries and individual products (refer to specific product guidelines).
Child: Dose is individualised based on the physical status of the patient, the vascularity of the oral tissues, the area of the oral cavity to be anaesthetised, and the technique of anaesthesia used. For oral infiltration or mandibular block: <10 years 0.9-1 mL; ≥10 years Same as adult dose. Max: Lidocaine 7 mg/kg. Dosage recommendations may vary among countries and individual products (refer to specific product guidelines).
Lidocaine 2% (20 mg) and epinephrine 1:100,000 (10 mcg) per mL solution for inj
Lidocaine 2% (20 mg) and epinephrine 1:80,000 (22.7 mcg) per mL solution for inj
Dose is individualised based on the physical status of the patient, the vascularity of the oral tissues, the area of the oral cavity to be anaesthetised, and the technique of anaesthesia used. For oral infiltration or mandibular block: 1-5 mL. Max: Lidocaine 7 mg/kg (not to exceed 200-500 mg, depending on the procedure and product used). Dosage recommendations may vary among countries and individual products (refer to specific product guidelines).
Child: Dose is individualised based on the physical status of the patient, the vascularity of the oral tissues, the area of the oral cavity to be anaesthetised, and the technique of anaesthesia used. For oral infiltration or mandibular block: <10 years 0.9-1 mL; ≥10 years Same as adult dose. Max: Lidocaine 7 mg/kg. Dosage recommendations may vary among countries and individual products (refer to specific product guidelines).
Parenteral
Infiltration anaesthesia
Adult: Available preparations:
Lidocaine 1% (10 mg) and epinephrine 1:200,000 (5 mcg) per mL solution for inj
Lidocaine 1.5% (15 mg) and epinephrine 1:200,000 (5 mcg) per mL solution for inj
Lidocaine 2% (20 mg) and epinephrine 1:200,000 (5 mcg) per mL solution for inj
Dose is individualised based on the procedure, degree and duration of anaesthesia required, vascularity of tissue and physical status of the patient. Max: Lidocaine 7 mg/kg. Dosage recommendations may vary among countries and individual products (refer to specific product guidelines).
Child: Infants, children and adolescents Dose is individualised based on the age and weight, procedure, degree and duration of anaesthesia required, vascularity of tissue and physical status of the patient. Max: Lidocaine 7 mg/kg. Dosage recommendations may vary among countries and individual products (refer to specific product guidelines).
Lidocaine 1% (10 mg) and epinephrine 1:200,000 (5 mcg) per mL solution for inj
Lidocaine 1.5% (15 mg) and epinephrine 1:200,000 (5 mcg) per mL solution for inj
Lidocaine 2% (20 mg) and epinephrine 1:200,000 (5 mcg) per mL solution for inj
Dose is individualised based on the procedure, degree and duration of anaesthesia required, vascularity of tissue and physical status of the patient. Max: Lidocaine 7 mg/kg. Dosage recommendations may vary among countries and individual products (refer to specific product guidelines).
Child: Infants, children and adolescents Dose is individualised based on the age and weight, procedure, degree and duration of anaesthesia required, vascularity of tissue and physical status of the patient. Max: Lidocaine 7 mg/kg. Dosage recommendations may vary among countries and individual products (refer to specific product guidelines).




 Đăng xuất
Đăng xuất